Note: This blog was written a few years ago and there have been changes to HIPAA audit protocols not reflected here. For more up-to-date information, check out our more recent blogs, or contact us for a quick call.
With the pinnacle of patient breaches hopefully behind us (e.g. Anthem/WellPoint breach, Premera, Blue Cross, and others in 2015), it is clear the industry has struggled with proper security of our electronic health information (ePHI). As such, the federal government has stepped in to ensure measures are in place to secure ePHI, abide by privacy rules granting all of us access to our health information, and making it illegal to discover a breach and not take appropriate steps to notify those affected.
What is the OCR HIPAA Audit Program?
The Office for Civil Rights (OCR) is a division of Health and Human Services with the responsibility to ensure industry compliance with an individual’s rights to Privacy, safeguards to electronic PHI and to investigate an organization’s diligence when breaches occur. Part of the OCR’s focus is also to develop audit rules in its activities ensuring the industry is adopting compliance efforts, reducing risk of breaches and improving health care. This is called the HIPAA Audit Program, and leverages the instructions, called the Audit Protocol, to test compliance.
Phases of the OCR’s HIPAA Audit Program
Phase 1 of the HIPAA Audit Program officially ended and Phase 2 of the HIPAA Audit program was announced on March 21, 2016 by Health and Human Services. In April 2016 they announced the updated HIPAA Audit Protocol. To clarify, the HIPAA law itself has not changed since the Omnibus update in 2013, but the government’s auditing of compliance has been updated and expanded.
The HIPAA Audit Protocol is something the Healthcare Information Technology compliance and audit communities have been asking for a long time, which is more guidance on HIPAA regulations. In addition to NIST-based risk analysis methodologies, this new set of protocols (instructions) are the most comprehensive guidance we have for HIPAA security (safeguards around electronic protected health information, or PHI), privacy (rights and restrictions to PHI) and breach notification requirements (what to do when a breach of PHI happens). This graphic shows the number of top-level HIPAA citations covered under the OCR’s checklist, color-coded by discipline:
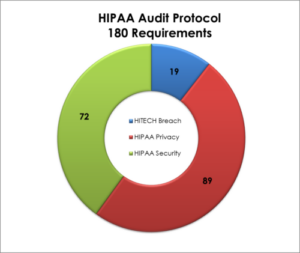
Summary of Phase 1 vs. Phase 2 of OCR’s HIPAA Audit Program
What it was – Phase 1 of the OCR’s Privacy, Security and Breach Notification Audit Program:
- HITECH added Breach Notification to HIPAA and endorsed the OCR‘s Audit Program.
- Contained 169 total protocols.
- Pilot program included 115 covered entities.
What it is now – the HIPAA Audit Program-Phase 2:
- OCR is implementing Phase 2 to include both CEs and business associates (every covered entity and business associate is eligible for an audit)
- Provides an opportunity for the OCR to identify best practices, risks and issues before they result in bigger problems (e.g. resulting in a breach) through the expanded random audit program.
- 180 Enhanced protocols (groups of instructions) which contain the following updates:
- Privacy – 708 updates (individual lines of instructions)
- Most notable changes are more policies and procedures surrounding the HIPAA Privacy Officer as well as some changes for Health Plans and Business Associates.
- Security – 880 updates (individual lines of instructions)
- Most notable changes are that Health Plans must have assurances from their plan sponsors and all companies now have to get proof of HIPAA compliance from their business associates, vendors and subcontractors.
- Privacy – 708 updates (individual lines of instructions)
The Current State of HIPAA Audit Checklists and Tools
With so many recent changes, it is clear that checklists, spreadsheets, the OCR’s SRA tool , HITRUST and most commercial compliance software companies are now out of date with the new HIPAA Audit Protocol. As we get to the end of the Meaningful Use incentive program, we risk having a high number of covered entities potentially using outdated software tools for modern HIPAA compliance requirements.
Regarding the HIPAA Audit Protocol’s compliance date, says Brad Trudell of MetaStar, “Remember it’s intended to detail the specific questions OCR plans to ask in Phase 2 audits to determine compliance with the previously existing HIPAA/HITECH requirements. If possible, CEs/BAs should use the protocol as the basis for conducting their own internal audits to make sure compliance is whipped into shape before the REAL auditors come knocking.”
In other words, the compliance date would match the release date – April of 2016 (about 2 months before this article was written).
Why invest in outdated Audit Protocol? HIPAA One® announced on June 15, 2016 they are current with the OCR’s Phase 2 of the Audit Program. To learn more about how your organization can simplify and automate HIPAA Security, Privacy and Breach Notification Assessments, Mock-Audits and Risk Analysis in compliance with the HIPAA Audit Protocol, HITECH and NIST-based methodologies, contact us.